
What Is Delta Tf in Chemistry
Overview. Tuberculosis (TB) is a serious illness that mainly affects the lungs. The germs that cause tuberculosis are a type of bacteria. Tuberculosis can spread when a person with the illness coughs, sneezes or sings. This can put tiny droplets with the germs into the air. Another person can then breathe in the droplets, and the germs enter.
√ Rumus Delta T
Temperature (T) = 80.0 K. Specific heat (c) = 1676 KJ. Now we have to convert the specific heat into Joules because it is in Kilojoules. So, the conversion is like this. 1 KJ = 1,000 J. So, 1676 KJ = 1,000 × 1676 = 16,76,000 J. Now put all the values in the formula. But, before that, we have to reorganize the formula to find specific heat.
cara menghitung rumus star delta kelistrikan YouTube
ΔTb = Tb larutan −Tb pelarut murni. Rumus di atas berlaku untuk larutan nonelektrolit. b. ΔTb tidak berlaku untuk larutan yang mudah menguap.. Misalkan = delta yaa Tb = m . Kb Tb/ m = kb Dengan: Tb = kenaikan titik didih m = molalitas kb = tetapan kenaikan titik didih #cmiiw 27 November 2020 pukul 00.32

Termokimia Pengertian Persaman Reaksi Rumus Dan Contoh Soal Soal Sexiz Pix
Dr. Hodge Sanitarium. Dr. Hodge Sanitarium, 424 Pine Ave., Niagara Falls, was operated by doctors William and John Hodge, who were brothers. They were Niagara County residents who grew up on a.

Rumus Cure Rate Tb Paru Indikator Nasional Penanggulangan Tb Kasus yang tidak memenuhi
So, from the first and 2nd laws of thermodynamics applied to this control volume system, $$\dot{q}-\dot{w}=\Delta h$$ and $$\frac{\dot{q}}{T}+\dot{\sigma}=\Delta s$$ where $\dot{q}$ is the heat flow per mole from the reservoir to the control volume, $\dot{w}$ is the shaft work per mole done on the surroundings, and $\dot{\sigma}$ is the entropy.
Model performance and delta with the 3layer DNN) Download Scientific Diagram
The Formula for Boiling Point. It are often calculated as: Kb = RTb2M/ΔHv, R is that the universal gas constant. Tb is that the boiling temperature of the pure solvent [in K] M is that the molar mass of the solvent. ΔHv is that the heat of vaporization per mole of the solvent. If the warmth of vaporization and therefore the vapor pressure of.

Mengenal Tuberkulosis TB Pada Anak, Ciri dan Gejala Dunia Biza
From thermodynamics, the heat capacity is defined as Cp = (dH dT)P C p = ( d H d T) P. That means we can calculate the heat required to change the temperature of some material from the following integral: H2 −H1 = Q = ∫T2 T1 Cp(T)dT H 2 − H 1 = Q = ∫ T 1 T 2 C p ( T) d T. In the range of 298-1200K, the heat capacity of CO 2 is given by.

Rumus Zscore TB/U & PB/U Informasi Seputar Gizi
Boiling Point Elevation. The boiling points of solutions are all higher than that of the pure solvent. Difference between the boiling points of the pure solvent and the solution is proportional to the concentration of the solute particles: ΔTb = Tb(solution) −Tb(solvent) = Kb × m (1) (1) Δ T b = T b ( s o l u t i o n) − T b ( s o l v e n.
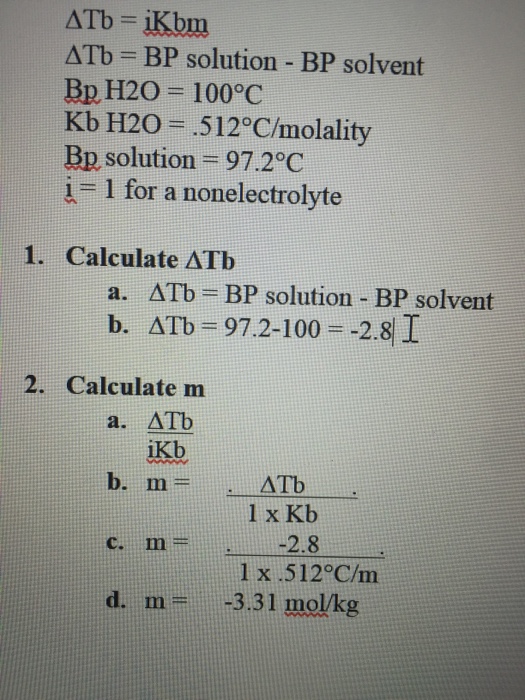
Solved Delta Tb = iKbm Delta Tb = BP solution BP solvent
ΔTb = m × Kb. Titik didih larutan = 100 °C + 5,2 °C = 105,2 °C. Titik beku larutan = 0 °C - 18,6 °C = -18,6 °C. 2. Titik beku larutan 64 gram naftalena dalam 100 gram benzena adalah 2,91 °C. Jika titik beku benzena 5,46°C dan tetapan titik beku molal benzena 5,1 °C, maka. tentukan massa molekul relatif naftalena! 3.

Rumus Kenaikan Titik Didih Hot Sex Picture
100, 0128 ∘ C. 5. 100, 0234 ∘ C. Belajar Modul, Rumus, & Soal Sifat Koligatif Larutan dan kuis interaktif. Dapatkan Modul, Rumus, & Soal Sifat Koligatif Larutan lengkap di Wardaya College.

√ Rumus Delta T
Absolute Delta. If you have a random pair of numbers and you want to know the delta - or difference - between them, just subtract the smaller one from the larger one. For example, the delta between 3 and 6 is (6 - 3) = 3. If one of the numbers is negative, add the two numbers together. The operation looks like this: (6 - {-3}) = (6 + 3) = 9.

√ Rumus Delta T
Hitunglah Disini yang pertama kenaikan titik didih larutan Kemudian yang kedua titik didih larutan tersebut dengan mr-nya 342 KB air = 0,512 derajat Celcius kg per mol atau = 0,52 derajat Celcius per molal air = 100 derajat Celcius Nah di sini sukrosa merupakan larutan nonelektrolit di mana nilainya atau faktor van't hoff nya = 1 Delta TB atau.
What is the need and applicability of Van't Hoff Factor? Explain the conditions where delta Tb
Berikut penjelasan kelompok sifat koligatif: 1. Koligatif Larutan - Penurunan tekanan uap jenuh (Δ Tp ) Penurunan tekanan uap jenuh adalah selisih tekanan uap pelarut murni dan tekanan uap larutan . tabel penurunan tekanan uap jenuh larutan non elektrolit dan elektrolit ↓. Uraian. Larutah non elektrolit.

√ Rumus Delta T
Rumus delta TB adalah rumus yang digunakan untuk menghitung perbedaan suhu badan atau temperatur tubuh. Rumus ini sangat penting untuk mengetahui seberapa besar perbedaan suhu badan seseorang yang sedang sakit atau dalam kondisi tertentu. Suhu badan normal manusia adalah sekitar 36,5-37,5 derajat Celsius. Namun, jika seseorang mengalami demam.

√ Rumus Delta T
بِسْــــــــــــــــــمِ اللهِ الرَّحْمَنِ الرَّحِيْمِ. Assalamualaikum w.r.b. teman-teman. Slamat datang dibisakimia.com insyaallah pasti bisa. Berikut ialah Rumus Serta Soal dan Pembahasan SIFAT KOLIGATIF LARUTAN. Jadi ini adalah bab I mapel kimia dikelas XII .
(PDF) The utility of delta neutrophil index in differentiation of pulmonary tuberculosis from
Meskipun sifat koligatif melibatkan larutan, sifat koligatif tidak bergantung pada interaksi antara molekul pelarut dan zat terlarut, tetapi bergatung pada jumlah zat terlarut yang larut pada suatu larutan. Sifat koligatif terdiri dari penurunan tekanan uap, kenaikan titik didih, penurunan titik beku, dan tekanan osmotik. 1. Penurunan Tekanan Uap.